Super Slueths
- Baylie Whiting, Alyssa Manwarring, Jake Clark
- Feb 24, 2016
- 3 min read
Thinking of being a forensic chemist? Let’s see if you’ve got the chops!
Here’s the set-up:
You need to identify which acid you are working with. We’ve narrowed it down to three choices for you.

4-(T-Octyl) Phenol
Stearic Acid
Palmitic Acid

We’ve done a lab with the mystery acid for you. This was the procedure followed:
About 3 mm of the mystery acid was collected in a capillary tube. The capillary tube was attached to a thermometer probe with a rubber band (See the picture on the right). A 250mL beaker was filled about ⅔ of the way full with water and put on a hot plate. The thermometer probe and capillary probe were suspended into the water using a clamp. The hot plate was turned on and a program was used to track the temperature of the water. As soon as the acid began to melt the temperature was noted. As soon as it was all melted the temperature was noted. All of this was repeated a second time for another test run.
This is the data collected from the procedure. You’ll notice the first test has less data because we missed the beginning of it starting to melt.
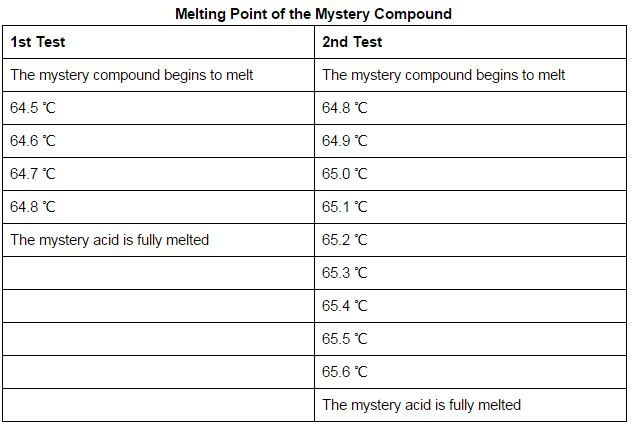
See video here!
Calculate the average melting point for each test.
Did you get these averages? If not back to the drawing board for you!
Pssst...if it’s been awhile since you’ve averaged something here’s a quick tip. All you have to do is add all the numbers together and divide it by however many numbers you added. Now go try again!
Test 1 Average: 64.65 ℃
Test 2 Average: 65.2 ℃
These melting points are all based off of Intermolecular Forces (IMF). IMF are the “sticky factor” between molecules. There are three kinds of forces: London Dispersion Forces, Dipole-Dipole, and Hydrogen Bonds. London Dispersion Force is very weak. Hydrogen Bonds are the strongest of all. Dipole-Dipole forces are not very strong or weak. Dipole-Dipole forces happen when the molecule has an uneven distribution of electrons. One side of the molecule has more electrons so it has a negative charge and so that side is attracted to another molecule’s more positive side. London’s Dispersion Force is so weak because the electrons are distributed evenly on the molecule. The electrons move around a little bit briefly creating negative and positive areas on the molecule that are attracted to another molecule for small amounts of time. Hydrogen bonds are very strong because they only bond with N, O, or F molecule which are very negative. The melting point is the point where these bonds start to break. So a molecule held together by London-Dispersion Force would melt easily while a Hydrogen bond would melt at a higher temperature.
Can you feel the excitement yet? We are moments away from discovering our mystery acid!
From our averages we can reason that the melting point for the acid is around the low to mid 60℃ range. Now this next part may seem to easy to be true. Just go google the melting points of each of our acid choices!
Did you find the same melting points as us?
Octyl) Phenol Acid- 79 ℃
Stearic Acid- 69.3 ℃
Palmitic Acid- 62.0 ℃
Octyl) Phenol Acid is clearly not a choice anymore. The final two choices are Stearic Acid and Palmitic Acid. The melting point average of the mystery acid was low to mid 60 ℃ range. This would make Palmitic Acid the most sensible choice!
Congrats! If you got Palmitic Acid as the mystery acid you might just have a future as a Forensic Chemist!
Comments